Abstract
Introduction
The direct oral anticoagulants (DOACs) including apixaban, dabigatran, edoxaban, and rivaroxaban are increasingly utilized for the management of venous thromboembolic disease (VTE) and/or non-valvular atrial fibrillation (NVAF). Adding aspirin (ASA) to warfarin or DOAC therapy increases bleeding risk. Patients on combination therapy with ASA and an anticoagulant were not well represented in clinical trials comparing DOACs to warfarin. We sought to compare bleeding and thrombotic outcomes with DOACs and ASA compared to warfarin and ASA in a non-trial setting.
Methods
We conducted a retrospective registry-based cohort study of adults on DOAC or warfarin therapy for VTE and/or NVAF. Warfarin treated patients were followed by six anticoagulation clinics. Four out of the six clinics contributed data on their patients that were on DOACs in the Michigan Anticoagulation Quality Improvement Initiative (MAQI 2) from January 2009 to June 2021. Patients were excluded if they had a history of heart valve replacement, recent myocardial infarction, or less than 3 months of follow-up. Two propensity matched cohorts (warfarin+ASA vs DOAC+ASA) of patients were analyzed based on ASA use at the time of study enrollment. The primary outcome was any new bleeding event. Secondary outcomes included new episodes of arterial or venous thrombosis, bleeding event type (major, fatal, life threatening, central nervous system, and non-major bleeding), emergency room visits, hospitalizations, transfusions, and death. Random chart audits were done to confirm the accuracy of the abstracted data. Event rates were compared using Poisson regression.
Results
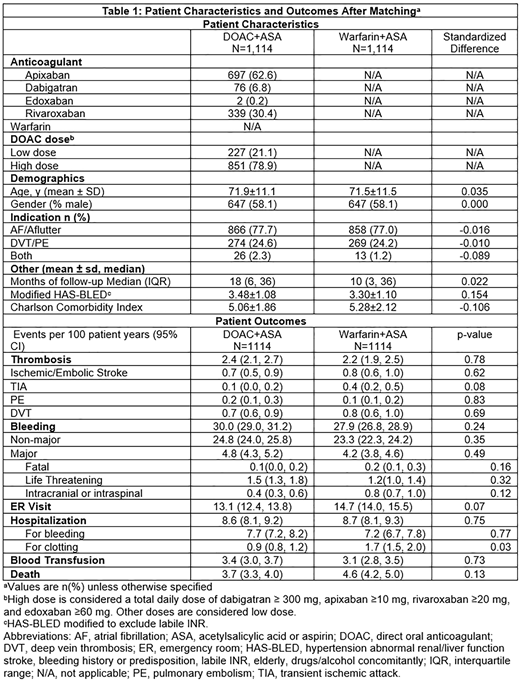
We identified a total of 1,139 patients on DOACs plus ASA and 4,422 patients on warfarin plus ASA. After propensity matching, we compared two groups of 1,114 matched patients. DOAC treated patients were predominately on apixaban (62.3%) and rivaroxaban (30.4%), most often at therapeutic doses (Table 1). Patients were largely (90.5%) on low dose ASA (≤ 100 mg). Patient demographics, co-morbidities, indication for anticoagulation, history of bleeding or clotting, medications, and duration of follow-up were well-balanced after matching. Patients were followed for a median of 11.7 months (interquartile range 4.4 and 34 months). Patients treated with DOAC+ASA had 2.4 thrombotic events per 100 patient years compared to 2.2 thrombotic events per 100 patient years with warfarin+ASA (P=0.78). There were no significant differences observed between groups by thrombotic subtype (stroke, transient ischemic attack, pulmonary embolism, deep vein thrombosis, table 1). Bleeding was also similar with 30.1 bleeding events per 100 patient years with DOAC+ASA compared to 27.8 bleeds per 100 patient years with warfarin+ASA (P=0.24). There were no significant differences by bleeding subtype (table 1). Hospitalizations for clotting occurred less frequently with DOAC+ASA (0.9 hospitalizations per 100 patient years) compared to warfarin+ASA (1.7 hospitalizations per 100 patient years, P=0.03). Mortality, transfusions, and healthcare utilization were otherwise similar between the two groups.
Conclusions
For patients on a DOAC versus warfarin with ASA for atrial fibrillation and/or venous thromboembolic disease without a recent myocardial infarction or heart valve replacement, bleeding and thrombotic outcomes were similar.
Kaatz: Gilead: Consultancy; CSL Behring: Consultancy; Novartis: Consultancy; Bristol Myer Squibb: Consultancy, Research Funding; Pfizer: Consultancy; Alexion: Consultancy; Janssen: Consultancy, Research Funding; Osmosis Research: Research Funding. Kline-Rogers: Janssen: Consultancy; American College of Physicians: Consultancy. Sood: Bayer: Consultancy. Froehlich: Merck: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Boehringer-Ingelheim: Consultancy; Pfizer: Consultancy; Blue Cross Blue Shield of Michigan: Research Funding; Fibromuscular Disease Society of America: Research Funding. Barnes: National Certification Board of Anticoagulation Providers: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Acelis: Consultancy; AMAG Pharmaceuticals: Consultancy; Connected Health: Consultancy; Blue Cross Blue Shield of Michigan: Research Funding; AC Forum: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Bristol-Myers Squibb: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal